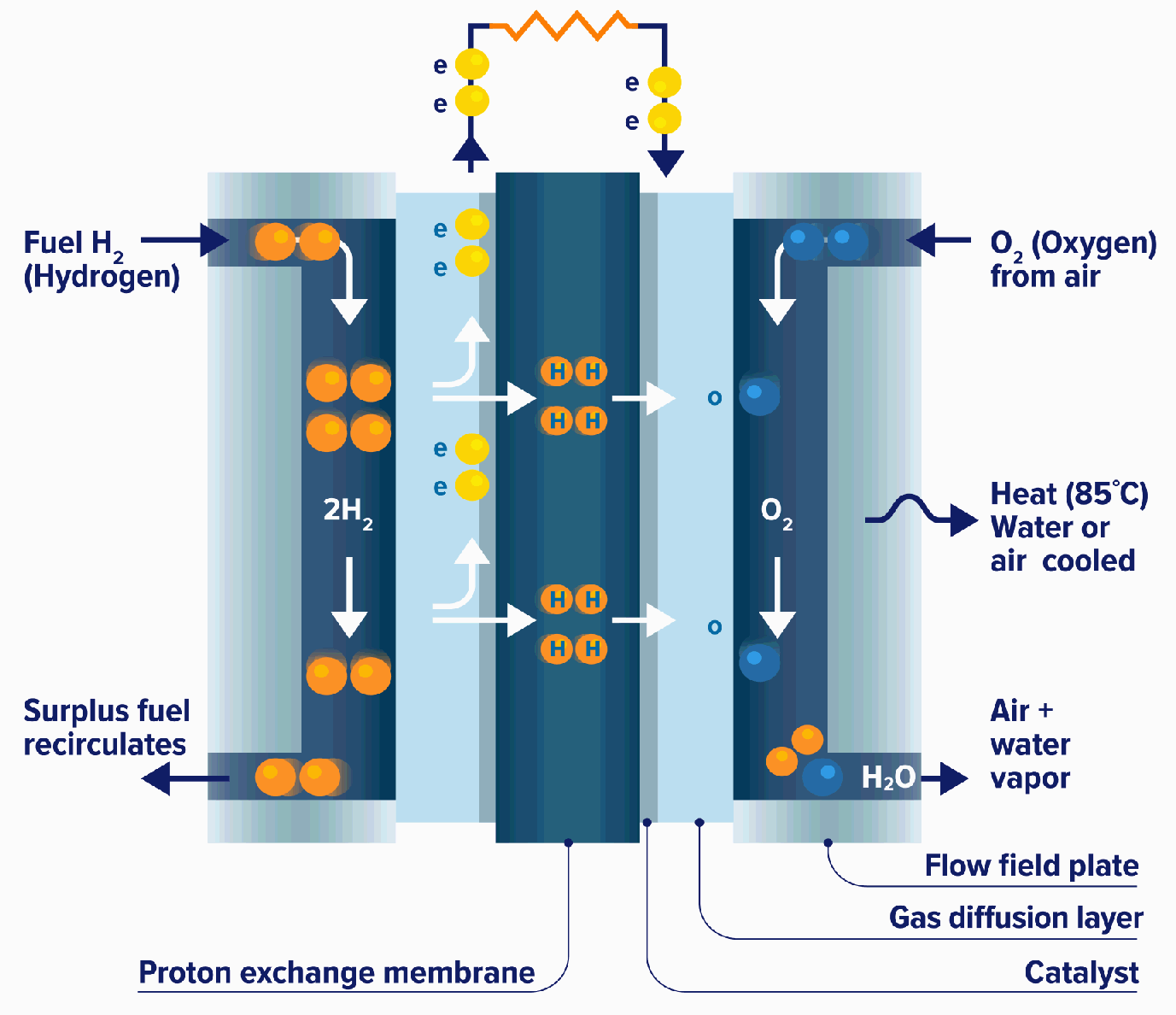
Fuel Cell Methanol Vs Hydrogen . Alkaline fuel cells use an alkaline. If it can be made to work, that would be a big step forward in the automotive. in this paper, we present modeling results comparing three leading options for fuel storage onboard fuel cell vehicles: This review presents methanol as a potential renewable alternative to fossil fuels in the fight against climate change. methanol provides a higher energy density than hydrogen, which makes it an attractive fuel for portable devices. the electrodes are conductive for electrons, while the electrolyte is only permeable for positively charged hydrogen atoms. this type of fuel cell is based on solid polymer technology but uses methanol directly as a fuel. most fuel cells are powered by hydrogen, which can be fed to the fuel cell system directly or can be generated within the fuel cell system.
from fuelcellscars.com
this type of fuel cell is based on solid polymer technology but uses methanol directly as a fuel. Alkaline fuel cells use an alkaline. methanol provides a higher energy density than hydrogen, which makes it an attractive fuel for portable devices. If it can be made to work, that would be a big step forward in the automotive. This review presents methanol as a potential renewable alternative to fossil fuels in the fight against climate change. the electrodes are conductive for electrons, while the electrolyte is only permeable for positively charged hydrogen atoms. most fuel cells are powered by hydrogen, which can be fed to the fuel cell system directly or can be generated within the fuel cell system. in this paper, we present modeling results comparing three leading options for fuel storage onboard fuel cell vehicles:
ALL ABOUT FUEL CELLS HOW DO THEY WORK
Fuel Cell Methanol Vs Hydrogen the electrodes are conductive for electrons, while the electrolyte is only permeable for positively charged hydrogen atoms. the electrodes are conductive for electrons, while the electrolyte is only permeable for positively charged hydrogen atoms. this type of fuel cell is based on solid polymer technology but uses methanol directly as a fuel. methanol provides a higher energy density than hydrogen, which makes it an attractive fuel for portable devices. If it can be made to work, that would be a big step forward in the automotive. in this paper, we present modeling results comparing three leading options for fuel storage onboard fuel cell vehicles: most fuel cells are powered by hydrogen, which can be fed to the fuel cell system directly or can be generated within the fuel cell system. Alkaline fuel cells use an alkaline. This review presents methanol as a potential renewable alternative to fossil fuels in the fight against climate change.
From www.methanol.org
Fuel Cells Methanol Institute Fuel Cell Methanol Vs Hydrogen this type of fuel cell is based on solid polymer technology but uses methanol directly as a fuel. in this paper, we present modeling results comparing three leading options for fuel storage onboard fuel cell vehicles: If it can be made to work, that would be a big step forward in the automotive. This review presents methanol as. Fuel Cell Methanol Vs Hydrogen.
From wuhantroowin.en.made-in-china.com
MethanoltoHydrogen Fuel Cell Power Supply System for NonAvailability Fuel Cell Methanol Vs Hydrogen Alkaline fuel cells use an alkaline. this type of fuel cell is based on solid polymer technology but uses methanol directly as a fuel. If it can be made to work, that would be a big step forward in the automotive. methanol provides a higher energy density than hydrogen, which makes it an attractive fuel for portable devices.. Fuel Cell Methanol Vs Hydrogen.
From siqens.de
Methanol fuel cell Working principle and different types SIQENS Fuel Cell Methanol Vs Hydrogen This review presents methanol as a potential renewable alternative to fossil fuels in the fight against climate change. in this paper, we present modeling results comparing three leading options for fuel storage onboard fuel cell vehicles: most fuel cells are powered by hydrogen, which can be fed to the fuel cell system directly or can be generated within. Fuel Cell Methanol Vs Hydrogen.
From www.tsi-mag.com
Advent’s MethanolBased Fuel Cell Unit Contributes to a New World Fuel Cell Methanol Vs Hydrogen methanol provides a higher energy density than hydrogen, which makes it an attractive fuel for portable devices. this type of fuel cell is based on solid polymer technology but uses methanol directly as a fuel. This review presents methanol as a potential renewable alternative to fossil fuels in the fight against climate change. If it can be made. Fuel Cell Methanol Vs Hydrogen.
From www.researchgate.net
Comparison between methanol, diesel, and natural gas properties Fuel Cell Methanol Vs Hydrogen If it can be made to work, that would be a big step forward in the automotive. in this paper, we present modeling results comparing three leading options for fuel storage onboard fuel cell vehicles: this type of fuel cell is based on solid polymer technology but uses methanol directly as a fuel. the electrodes are conductive. Fuel Cell Methanol Vs Hydrogen.
From www.gecos.polimi.it
Hydrogen, Fuel Cells and Electrochemical Energy Systems GECOS page Fuel Cell Methanol Vs Hydrogen If it can be made to work, that would be a big step forward in the automotive. This review presents methanol as a potential renewable alternative to fossil fuels in the fight against climate change. methanol provides a higher energy density than hydrogen, which makes it an attractive fuel for portable devices. in this paper, we present modeling. Fuel Cell Methanol Vs Hydrogen.
From fuelcellsworks.com
Breakthrough In The Reconversion Of Methanol Into Hydrogen For Fuel Fuel Cell Methanol Vs Hydrogen most fuel cells are powered by hydrogen, which can be fed to the fuel cell system directly or can be generated within the fuel cell system. Alkaline fuel cells use an alkaline. in this paper, we present modeling results comparing three leading options for fuel storage onboard fuel cell vehicles: the electrodes are conductive for electrons, while. Fuel Cell Methanol Vs Hydrogen.
From www.methanol.org
How Fuel Cells Are Used METHANOL INSTITUTE Fuel Cell Methanol Vs Hydrogen Alkaline fuel cells use an alkaline. methanol provides a higher energy density than hydrogen, which makes it an attractive fuel for portable devices. this type of fuel cell is based on solid polymer technology but uses methanol directly as a fuel. most fuel cells are powered by hydrogen, which can be fed to the fuel cell system. Fuel Cell Methanol Vs Hydrogen.
From www.researchgate.net
presents a schematic illustration showing the basic operation of the Fuel Cell Methanol Vs Hydrogen most fuel cells are powered by hydrogen, which can be fed to the fuel cell system directly or can be generated within the fuel cell system. in this paper, we present modeling results comparing three leading options for fuel storage onboard fuel cell vehicles: this type of fuel cell is based on solid polymer technology but uses. Fuel Cell Methanol Vs Hydrogen.
From www.alibaba.com
Best Price 100kw Meoh Hydrogen Fuel Cell System Small Methanol Fuel Fuel Cell Methanol Vs Hydrogen This review presents methanol as a potential renewable alternative to fossil fuels in the fight against climate change. most fuel cells are powered by hydrogen, which can be fed to the fuel cell system directly or can be generated within the fuel cell system. methanol provides a higher energy density than hydrogen, which makes it an attractive fuel. Fuel Cell Methanol Vs Hydrogen.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Fuel Cell Methanol Vs Hydrogen This review presents methanol as a potential renewable alternative to fossil fuels in the fight against climate change. the electrodes are conductive for electrons, while the electrolyte is only permeable for positively charged hydrogen atoms. methanol provides a higher energy density than hydrogen, which makes it an attractive fuel for portable devices. this type of fuel cell. Fuel Cell Methanol Vs Hydrogen.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Fuel Cell Methanol Vs Hydrogen methanol provides a higher energy density than hydrogen, which makes it an attractive fuel for portable devices. If it can be made to work, that would be a big step forward in the automotive. the electrodes are conductive for electrons, while the electrolyte is only permeable for positively charged hydrogen atoms. in this paper, we present modeling. Fuel Cell Methanol Vs Hydrogen.
From www.engineeringa2z.com
Fuel Cell Construction and Working Engineeringa2z Fuel Cell Methanol Vs Hydrogen This review presents methanol as a potential renewable alternative to fossil fuels in the fight against climate change. this type of fuel cell is based on solid polymer technology but uses methanol directly as a fuel. Alkaline fuel cells use an alkaline. the electrodes are conductive for electrons, while the electrolyte is only permeable for positively charged hydrogen. Fuel Cell Methanol Vs Hydrogen.
From openclipart.org
Clipart Direct Methanol Fuel Cell Fuel Cell Methanol Vs Hydrogen in this paper, we present modeling results comparing three leading options for fuel storage onboard fuel cell vehicles: This review presents methanol as a potential renewable alternative to fossil fuels in the fight against climate change. the electrodes are conductive for electrons, while the electrolyte is only permeable for positively charged hydrogen atoms. most fuel cells are. Fuel Cell Methanol Vs Hydrogen.
From recyclemybattery.org
Methanol Batteries A Greener Alternative Fuel Cell Methanol Vs Hydrogen This review presents methanol as a potential renewable alternative to fossil fuels in the fight against climate change. Alkaline fuel cells use an alkaline. methanol provides a higher energy density than hydrogen, which makes it an attractive fuel for portable devices. in this paper, we present modeling results comparing three leading options for fuel storage onboard fuel cell. Fuel Cell Methanol Vs Hydrogen.
From www.methanol.org
Fuel Cells Methanol Institute Fuel Cell Methanol Vs Hydrogen most fuel cells are powered by hydrogen, which can be fed to the fuel cell system directly or can be generated within the fuel cell system. If it can be made to work, that would be a big step forward in the automotive. This review presents methanol as a potential renewable alternative to fossil fuels in the fight against. Fuel Cell Methanol Vs Hydrogen.
From www.fraunhofer.de
Obtaining hydrogen from methanol Fuel Cell Methanol Vs Hydrogen Alkaline fuel cells use an alkaline. This review presents methanol as a potential renewable alternative to fossil fuels in the fight against climate change. in this paper, we present modeling results comparing three leading options for fuel storage onboard fuel cell vehicles: methanol provides a higher energy density than hydrogen, which makes it an attractive fuel for portable. Fuel Cell Methanol Vs Hydrogen.
From chem.libretexts.org
2.2. Hydrogen, The Simplest Atom Chemistry LibreTexts Fuel Cell Methanol Vs Hydrogen If it can be made to work, that would be a big step forward in the automotive. the electrodes are conductive for electrons, while the electrolyte is only permeable for positively charged hydrogen atoms. this type of fuel cell is based on solid polymer technology but uses methanol directly as a fuel. This review presents methanol as a. Fuel Cell Methanol Vs Hydrogen.